TMS Treatments
For Depression
Innovative Therapy Changes Brain Activity
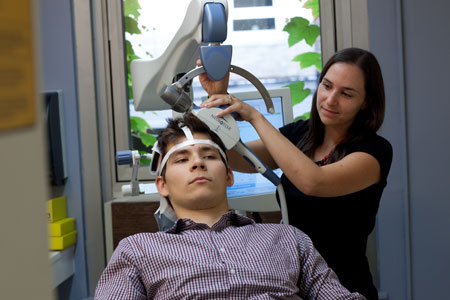
Transcranial Magnetic Stimulation (TMS) is an innovative therapy for those who have moderate to severe depression that has not responded to medication. TMS works by using magnetic fields to change specific patterns of brain activity. Using TMS allows doctors to alter brain activity without surgery and with minimal discomfort to the patient.
We conducted the first double blind clinical trial of TMS in depression and in our clinical program have successfully treated many patients who had not responded to other approaches. Patients can derive a sustained improvement in their depression, enabling more fulfilling lives.
Approved by the U.S. Food and Drug Administration (FDA)
The FDA has cleared a number of devices and treatment protocols for use in the treatment of depression in individuals who have not benefited from prior antidepressant medication. We offer treatment with a variety of these approved machines.The Berenson-Allen Center for Noninvasive Brain Stimulation is proud to have been the first to offer this therapy to patients in the Boston area.
For Other Indications
Brain stimulation techniques such as TMS and tDCS can be applied to different brain areas with the goal of treating other conditions. Research is ongoing to determine whether these techniques are effective to treat certain kinds of chronic pain, epilepsy, and motor conditions like ataxia. The Food and Drug Administration (FDA) has not yet approved the use of TMS or tDCS for these conditions. However, TMS and tDCS can sometimes be used as "off-label" treatments when deemed clinically appropriate, though they are considered investigational (not proven). As such, for any of these treatment indications, we do a complete neurological evaluation with all prospective patients to ensure there are no safety contraindications, and acquire informed consent to ensure that all patients are aware that the treatments are considered off-label.

